Europe and MEA Culture Test for Healthcare-Associated Infections Market to Reach USD 370.3 Million by 2032, Expanding at a CAGR of 3.1%
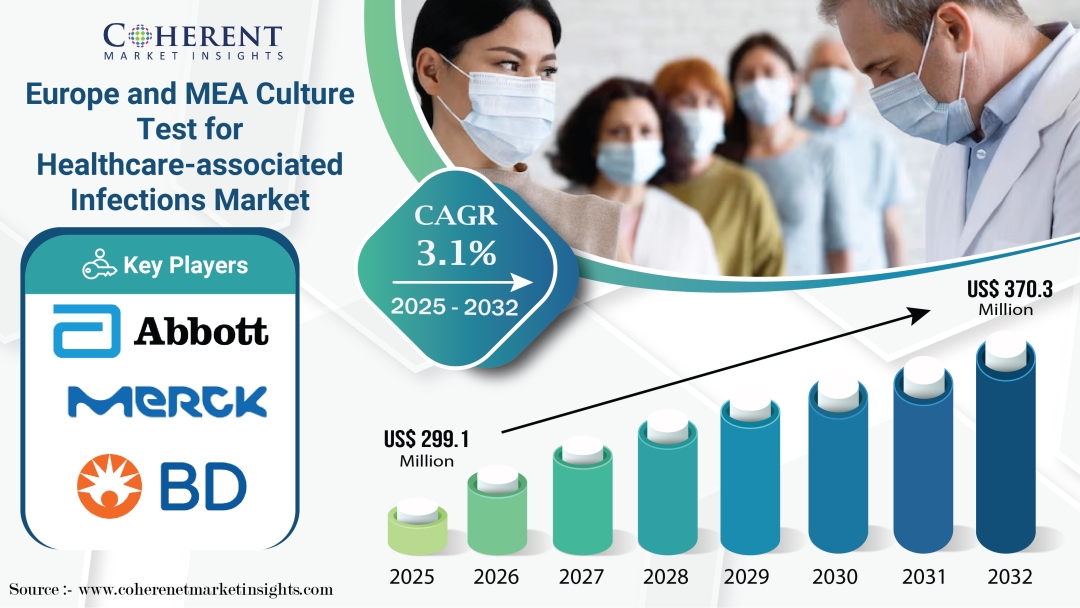
According to a recent report by Coherent Market Insights, the Europe and MEA Culture Test for Healthcare-associated Infections Market is estimated to be valued at USD 299.1 Mn in 2025 and is expected to reach USD 370.3 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 3.1% from 2025 to 2032. Over the projected period, the Europe and MEA culture test for healthcare-associated infections market is expected to show modest increase. The increasing dominance of hospital-acquired infections, such as pneumonia, urinary tract infections, and bloodstream infections, is expected to drive the demand for culture tests for early diagnosis and management.
Request for Sample Report: https://www.coherentmarketinsights.com/insight/request-sample/7750
Europe and MEA Culture Test for Healthcare-Associated Infections Market Key Takeaways
According to Coherent Market Insights (CMI), the Europe and MEA culture test for healthcare-associated infections market size is estimated to reach USD 299.1 Mn in 2025 and USD 370.3 Mn by 2032.
The target market will likely exhibit a moderate CAGR of 3.1% during the assessment period.
By product, instruments segment is expected to account for more than half of the Europe and MEA culture test for healthcare-associated infections market share in 2025.
Based on test type, bacterial culture test segment is projected to dominate the industry, holding a market share of 1/3 in 2025.
By application, surgical site infections category will likely account for around USD 80.75 Mn by 2025.
Rising Prevalence of Hospital-associated Infections Fueling Market Growth
Coherent Market Insights’ Europe and MEA culture test for healthcare-associated infections market report highlights key factors driving the industry’s growth. Increasing prevalence of hospital-associated infections (HAIs) like surgical site infections and urinary tract infections is one such prominent factor.
Hospital-acquired infections (HAIs) or nosocomial infections are a significant cause of morbidity and mortality worldwide. According to the World Health Organization (WHO), hundreds of millions of patients are affected by HAIs each year.
These infections often require accurate diagnosis through cultural tests to initiate timely treatment. Therefore, rising incidence of HAIs is expected to play a key role in boosting Europe and MEA culture test for healthcare-associated infections market growth.
Shift Towards Rapid Diagnostics Techniques Restraining Market Growth
The future Europe and MEA culture test for healthcare-associated infections market outlook looks bright. However, growing adoption of rapid diagnostic techniques is expected to limit market growth to some extent.
Healthcare professionals and patients across Europe and MEA are increasingly turning to faster molecular diagnostics like NGS and PCR. This is due to their increased accuracy, speed, and ability to detect a wide range of pathogens.
Traditional culture tests can take 24 to 72 hours or longer to yield results. This delay is encouraging a shift towards rapid diagnostic tests like PCR, leading to reduced Europe and MEA culture test for healthcare-associated infections market demand.
Purchase Now Up to 25% Discount on This Premium Research Report: https://www.coherentmarketinsights.com/insight/buy-now/7750
Rising Adoption of Infection Control Measures Creating Growth Opportunities
Both public and healthcare professionals across Europe and the MEA are increasingly recognizing the importance of robust infection control measures. This growing awareness is fueling demand for hospital-associated infection diagnostics like culture tests.
Europe has robust infection control regulations and policies requiring routine screening and quick diagnosis of HAIs. Many MEA countries are adopting similar European-inspired strategies to improve infection control and tackle HAIs and antimicrobial resistance. This will create lucrative growth opportunities for culture test product manufacturers in these regions.
Impact of AI on Europe and MEA Culture Test for HAIs Market
Artificial intelligence (AI) is significantly influencing culture test for healthcare-associated infections (HAIs) market in Europe and MEA. It helps companies enhance diagnostic speed, accuracy, and workflow efficiency.
AI-powered tools are being introduced in European microbiology labs to automate culture analysis. These technologies help reduce human error and enable faster detection of pathogens responsible for HAIs. Similarly, in MEA, AI adoption is gradually improving diagnostic infrastructure, supporting early outbreak detection.
A notable example of this trend is the launch of AI-based WASPLab® automation system by Copan Diagnostics in 2024. This system uses AI algorithms to analyze bacterial cultures and identify HAI-related pathogens with minimal human intervention.
Emerging Europe and MEA Culture Test for Healthcare-associated Infections Market Trends
Growing popularity of point-of-care culture testing is a key growth-shaping trend. To capitalize on this trend, industry players are focusing on developing faster culture techniques as well as miniaturized culture systems.
Introduction of automated culture testing systems is also supporting market expansion. Leading companies are integrating AI and automation in culture diagnostics to improve accuracy and turnaround time. This is making culture tests more efficient and appealing, leading to higher market demand.
There is a growing trend of integrating traditional culture tests with molecular assays to overcome diagnostic limitations and provide more rapid and accurate detection of HAIs. Adoption of these hybrid testing approaches is expected to boost Europe and MEA culture test for healthcare-associated infections market value.
Analyst’s View
“The Europe and MEA culture test for healthcare-associated infections market is poised to record moderate growth, owing to rising prevalence of hospital-acquired infections and adoption of effective infection control measures,” said senior analyst Manisha Vibhute.
Current Events and Their Impact on the Europe and MEA Culture Test for Healthcare-associated Infections Market
|
Event |
Description and Impact |
|
Rise in Nosocomial Outbreaks |
|
|
Antimicrobial Resistance (AMR) Declared a Top Public Health Threat |
|
|
Launch of AI-powered Culture Testing Platforms |
|
Competitor Insights
Key companies listed in Europe and MEA culture test for healthcare-associated infections market research report:
– Merck KGaA
– Abbott Laboratories
– Thermo Fisher Scientific Inc.
– Becton Dickinson and Company
– Hologic, Inc.
– Steris Plc
– Danaher Corporation
– F. Hoffmann-La Roche AG
– bioMérieux SA
– Siemens Healthineers AG
– Hardy Diagnostics
– QIAGEN N.V.
– Bio-Rad Laboratories, Inc.
– Cepheid
– SSI Diagnostica A/S
Key Developments
In April 2025, Merck announced plans to invest over € 300 million in new research science center at its headquarters in Germany.
In May 2024, Siemens Healthineers launched four novel blood biomarkers for a fully automated inflammation testing panel of seven immunoassays with less turnout.
In April 2025, Qiagen announced plans to introduce three new sample preparation instruments between 2025 and 2026 to strengthen its portfolio of automated sample preparation.
Market Segmentation
- Product Type Insights (Revenue, USD Mn, 2020 – 2032)
- Instruments
- Automated Culture Systems
- Incubators
- Analyzers
- Reagents and Consumables
- Culture Media
- Test Kits
- Antimicrobial Susceptibility Testing Kits
- Instruments
- Test Type Insights (Revenue, USD Mn, 2020 – 2032)
- Bacterial Culture Tests
- Aerobic Bacteria
- Anaerobic Bacteria
- Fungal Culture Tests
- Viral Culture Tests
- Mixed Infection Tests
- Application Insights (Revenue, USD Mn, 2020 – 2032)
- Surgical Site Infections
- Bloodstream Infections
- Urinary Tract Infections
- Respiratory Infections
- Gastrointestinal Infections
- Sample Type Insights (Revenue, USD Mn, 2020 – 2032)
- Blood
- Urine
- Stool
- Tissue
- Others
- End User Insights (Revenue, USD Mn, 2020 – 2032)
- Hospitals & Clinics
- Nursing Homes
- Diagnostic Laboratories
- Research Institutions
- Ambulatory Surgical Centers
- Others
Get Customization on this Report: https://www.coherentmarketinsights.com/insight/request-customization/7750
About Us:Coherent Market Insights leads into data and analytics, audience measurement, consumer behaviors, and market trend analysis. From shorter dispatch to in-depth insights, CMI has exceled in offering research, analytics, and consumer-focused shifts for nearly a decade. With cutting-edge syndicated tools and custom-made research services, we empower businesses to move in the direction of growth. We are multifunctional in our work scope and have 450+ seasoned consultants, analysts, and researchers across 26+ industries spread out in 32+ countries.
Media Contact
Company Name: Coherent Market Insights
Contact Person: Mr. Raj Shah
Email: Send Email
Phone: United states
Address:533 Airport Boulevard, Suite 400, Burlingame, CA 94010, United States
City: Burlingame
State: California
Country: United States
Website: https://www.coherentmarketinsights.com/industry-reports/europe-and-mea-culture-test-for-healthcare-associated-infections-market